美国“净网”:放弃美国、中国出海游戏会损失多少?
近,因为美国对TikTok的强买强卖引发巨大争议后,外界普遍开始担忧,美国政府将会发起更多针对 互联网企业的不利举动。这份担忧很快成真,昨天,美国国务卿蓬佩奥在华盛顿召开新闻发布会,威胁在美封禁阿里、腾讯、百度、 电信、 移动等 企业的云服务。
Recently, after the huge controversy caused by the strong buying and selling of tiktok in the United States, the outside world generally began to worry that the U.S. government will launch more adverse actions against Chinese Internet enterprises. Yesterday, US Secretary of state pompeio held a news conference in Washington, threatening to ban the cloud services of Chinese enterprises such as Alibaba, Tencent, Baidu, China Telecom and China Mobile in the United States.
随后,美国政府网站发布了蓬佩奥签名的 声明,提出了美国版的净网行动,包括五项具体工作,三项严格意义上与软件有关。指向不仅仅是稍微宽泛一点的 互联网企业,一直努力经营近两年的 游戏厂商,也有可能成为美国的净网狩猎目标。

游戏厂商,特别是有出海业务、尤其在美国有业务的企业,是时候警惕起来了。
另外,在美国“净网”的极限施压下,假如完全失去美国手游市场, 游戏企业将承受多大的损失呢?这是很多投资者关注的问题,今天GameLook就来简单分析一二。
美网要干净,干净的标准?没说
据声明,净网是美国之前提出的5G清洁路径的延续。所谓5G清洁路径,就是蓬佩奥之前5月提出的,督促美国盟国不使用华为、中兴等不可靠卖家提供的任何5G设备。
从性质上看,“净网”不仅是“5G清洁路径”覆盖面广度的延续,同样也是由硬件到软件的深度的延续。
具体而言,根据美方开列的清单,美方“净网”提出了五条新标准:


从细节上看,与国内游戏厂商有关的主要是2、3、4三点。第2点有可能导致国产游戏从App Store、Google Play上下架,考虑到声明中的说法是“美国应用商店”(US.mobile app stores),而不是“在美国地区的应用商店”(mobile app store in US.),而且所谓“干净的标准”不明,极限假设下,不排除美国会借机扩大化,比如不仅是从App Store、Google Play美国区下架,还有可能涉及美国的盟国,乃至全球市场的可能性。
当然,这也涉及到美国互联网公司的利益,因为苹果和谷歌也将从 游戏收入中分享。比如2019年,苹果AppStore只在 市场赚了100多亿美元,苹果不会轻易放弃这部分收入,所以可能性很低。
相对而言,第3点主要针对的是硬件厂商,以及要求美国开发者从华为应用商店如AppGallery中下架,执行力度反而更大。云服务的波及面,则可能对有云服务业务的大厂有所影响。
收集美国用户隐私信息?美国科技公司才是真正问题所在
虽然众所周知,为了达成目标,美国政府已经不再顾及颜面,往往“欲加之罪何患无辞”,不过还是可以仔细分析,目前 游戏出海是否符合美国“净网”定点打击的标准。
单从信息收集出发,就GameLook所知, 游戏厂商出海美国市场,高度依赖当地应用商店分发,以及Facebook、谷歌的营销服务,简单而言即买量。
此外,由于绝大多数 制造商在海上实行本地化,大多数海上游戏产品使用Facebook、苹果和谷歌。要收集美国用户的个人信息,更多的是美国本地广告平台、社交媒体甚至美国平台巨头做的更多,因为他们需要分析客户的偏好,以达到准确的交付,为 游戏公司提供更好的购买服务。
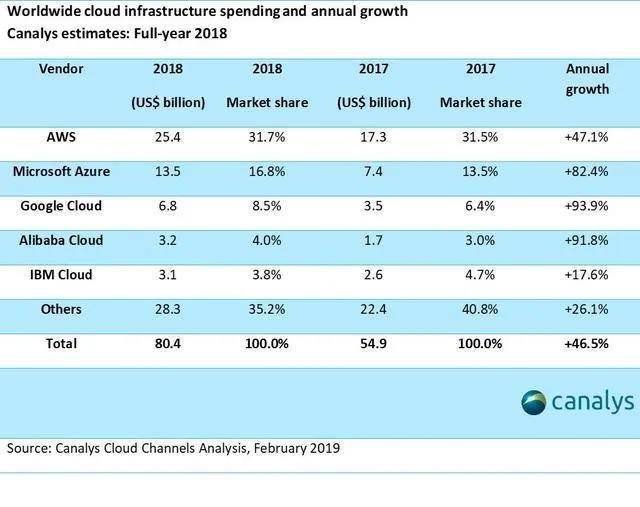
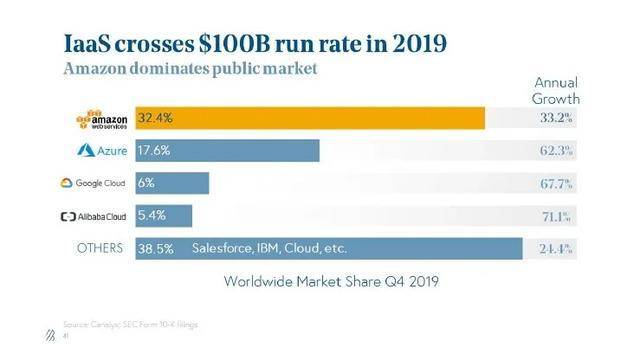
就云服务而言,提供云服务的 制造商在美国的市场份额也是有限的。根据多份报告,目前世界上有亚马逊AWS、微软Azure和阿里云三大云服务提供商。目前,许多 游戏企业在国外市场使用亚马逊、微软和谷歌云服务。
据SynergyResearch统计,近年来亚马逊AWS一直处于领先地位,占全球云服务市场份额的40%。虽然阿里巴巴云也很强大,但主要战场在亚太和 市场。领先的阿里巴巴云对美国本土云服务市场没有太大威胁,更不用说百度、腾讯、被美国严格防守的华为了。
就数据而言,出海美国的 游戏厂商,更有可能接触到用户的付费信息,以及无关痛痒的游戏中的八卦聊天和好友信息,而国外用户的信用卡早已被苹果、Google的平台所绑定,这一敏感数据 游戏公司一方面无法获得,另一方面也受到了苹果和谷歌的严格监管。
恐怕真正会引发隐私问题的游戏会上升到基于LBS地理位置的游戏产品,比如PokémonGO。但问题是,PokémonGO开发商Niantic是标准的美国企业,目前在美国还没有类似的 LBS游戏。
显然从用户数据搜集上,事情显然又饶了回去: 游戏所能搜集的美国用户信息之与游戏运营有关,真正高度敏感的用户隐私数据都被美国企业自己包圆了。
“懂王”要扣这个帽子给 游戏公司, 厂商可以顺手这顶帽子交给苹果、google、Facebook、亚马逊,他们才是美国用户隐私问题的关键所在。
仍要准备迎接“ 坏的结果”
前面提到的,美国政府早就不顾行为的正当性,从强迫TikTok卖身、抽取佣金的行为来看,美国政府已经打破了现代文明、现代商业社会的底线,奉行以自我为中心的 原始的巧取豪夺,所谓隐私只不过是冠冕堂皇的借口。所以,即使 游戏制造商有意识地行事正当,没有不恰当之处,他们仍然需要考虑 坏的结果。
坏的结果有多坏?数据可能 为直观,GameLook就来计算下失去美国市场 游戏公司可能会有多少损失。
据SensorTower统计,今年 季度,美国手游市场的收入为45.4亿美元。在这些产品中, 手游在美国市场的表现越来越突出,其中19个 产品进入了美国手游市场的TOP100,共吸收了4.87亿美元,比上一期增长了15.9%,占Top100总收入的16.3%。
且值得注意的是,从美国成绩突出的手游产品来看,只有6家上市游戏企业,很多 上市游戏厂商尚未在美国取得很大规模的收入。
单季度 游戏厂商在美国创收4.87亿美元约合33.8亿人民币,假设剩余三个季度收入相同,那就是一年135.2亿人民币。
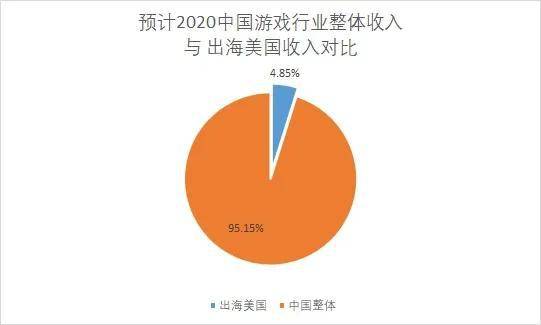
根据游戏工委《2020年1—6月 游戏产业报告》,今年上半年国内市场营销收入1394.93亿元,其中手游达1046.73亿元。 游戏企业上半年海外市场营销收入75.89亿美元(约合533.62亿元人民币)。假设下半年国内游戏业收入相同,2020年国内游戏市场收入约为2789.86亿元,海外市场收入1067.24亿元。
换言之,如果 坏情况发生,所有 手机游戏必须撤离美国市场,造成的损失约是整个国内游戏市场5%的收入规模损失,以及海外市场约13%的规模损失。
这种影响说大也大,说小也小,主要是对于那些拥有海外业务,并且更依赖美国海外业务的 制造商来说。例如腾讯主要是《PUBGM》和《CODM》,而《CODM》幸运的是,后者是通过动视暴雪,即美国本地企业发行的。网易相对来说,主要盘踞日本的网易,受影响相对较小。
而对于莉莉丝、FunPlus、龙创悦动等在美国收入较多的出海游戏公司来说,潜在影响 大。
危机迫近,锦囊妙计存在么?
这也牵扯了另一个问题,如果美国政府继续不讲道理、执意搞事,出海的 游戏厂商是否有应对方法,避免坐以待毙。
游戏产品显然比不上TikTok在美国的巨大影响力,不太可能上演“强买强卖”的戏码,如果美方把 游戏强行拉上赌桌,游戏产品在美国被封禁的可能性更高。
对于美方清单来看,GameLook认为从合规性考虑, 游戏公司可以通过寻找美国游戏发行商 、或在美国或其他 设立海外发行主体的方式,继续合法在美国市场运营。从风险来看,找美国 商的做法比较保险;而搞马甲主体的方式客观说只适用于小型出海发行商,对大型游戏公司来说、存在暴露后招致美方更疯狂打击的可能性。
长期来看,将业务重心放在 市场,跟随 政策进军“一带一路” 市场,以及关注非美国的海外市场是更为稳妥的方案。
不过,出海游戏公司回到 市场注定会迎来更为激烈的竞争,但如果成功赶上“内循环”的政策红利,押中 经济新一轮增长也不失为一波机遇。
“不讲道理”的美国市场,与美国政府宣扬的完全自由的市场经济,被其亲手撕碎有莫大关系,好在世界并不只有北美。同时这一系列事件也告诫游戏厂商, 化思维没错,不过无论海外市场多么光鲜亮丽,没有扎实的 市场基本盘,仅依靠出海战略显然在未来动荡的 环境下风险是颇大的。
The "unreasonable" U.S. market has a lot to do with the completely free market economy advocated by the U.S. government. Fortunately, North America is not the only one in the world. At the same time, this series of events also warned game manufacturers that it is right to think internationally, but no matter how bright the overseas market is, there is no solid basic market in China. It is obvious that relying on the strategy of going out to sea alone is quite risky in the turbulent international environment in the future.
如何使用Twitter推广你的企业品牌(上篇)

Twitter是一个简洁明了的信息通讯专用工具,容许您向定阅者(关注者)推送将近140字符的信息。您的Twitter能够包括偏向一切web內容(网络文章、网页页面、PDF文档等)或相片或视頻的连接。假如一张图片胜于万语千言,那麼在Twitter中加上一个照片会巨大地拓展你能共享的內容,足够超出Twitter的140字符的表述限定。大家关注(定阅)你的Twitter帐户,你也关注他人。这能够使你阅读文章、回应并轻轻松松地与你的跟随者共享或分享。
…


